HEMPZORB™ BIOAVAILABILITY, ABSORPTION
AND WATER SOLUBILITY
White Paper prepared by Med 7, llc contributing authors: E. Masters MD, P. Davis PDF,
D.A. Vontrap PHD, Mark Dente RN
Introduction: Understanding the bioavailability of CBD oil and all the Phytocannabinoids contained in full spectrum oil is like navigating ever changing sandbars in an uncharted river. Terms like “Pharmacokinetics,” “bioavailability,” “absorption” and “water solubility” are tossed around and used interchangeably. While similar, and related, they are not the same and should not be used interchangeably. By relying upon published and validated studies and recent replicated studies, we all can get an understanding that will help us avoid some of the misleading graphs and tables that are not supported by studies. We will focus on Cannabis sativa since this is the most common strain of cannabis in products today. The same principles and data should translate to other cannabis species.
CBD and THC: Cannabis sativa is species of herb which has been grown and cultivated over thousands of years for uses in clothing, textiles, and paper from its fibers and medicinally and recreationally from its oils and resins. These oils and resins are lipophilic or fat soluble. There are well over 100 chemical compounds found in the Cannabis sativa or hemp plant. These chemical compounds are known as cannabinoids. Because they are derived from a plant source, they are also called “Phytocannabinoids.” The most notable and well studied cannabinoids are THC (tetrahydrocannabinol) Cannabidiol (CBD). THC, specifically THC▲9, is responsible for the euphoric, mind-altering effects of cannabis and is the primary composition of marijuana due to its high THC▲9. CBD, on the other hand, is a non-psychoactive component of the cannabis sativa plant. Cannabidiol is a pleiotropic drug in that it produces many effects through multiple molecular pathways. The cannabis plant is also composed of a chemical mixture that includes other Phytocannabinoids, terpenes, flavonoids, steroids and enzymes. While the exact mechanism of action are not fully known, all of these other components have a synergistic effect combined with cannabinoids (Gallily, 2015). This has become known as the entourage effect. The cannabis plant has been consumed by humans for thousands of years in medicine for its sedative, antidepressant, analgesic, anticonvulsant, antiemetic, and anti-inflammatory effects.
CBD is an antagonists of cannabinoid receptors and a modulator of the endogenous cannabinoid system which has led to it being a promising candidate for clinical research and therapeutic uses. Cannabinoid receptors including CB1 and CB2 are distributed in the central nervous system and many peripheral tissues e.g. spleen, leukocytes; reproductive, urinary and gastrointestinal tracts; endocrine glands, arteries and heart, etc. (Pertwee, 2008). Additionally, there is now evidence for non-receptor dependent mechanisms of cannabinoids. Five endogenous cannabinoids, anandamide, 2-arachidonylglycerol, noladine ether, virodhamine, and NADA, have been detected and studied. There is also evidence that besides the two cannabinoid receptor subtypes cloned so far, additional cannabinoid receptor subtypes and vanilloid receptors are involved in the complex physiological functions of endocannabinoids that include, for example, motor coordination, memory procession, pain modulation and neuroprotection (Grotenhermen, 2004).
The Physical Properties of CBD and THC Oil: As mentioned above, both THC and CBD are highly lipophilic and have poor oral bioavailability. The oral bioavailability of CBD and THC has been well documented between 4-12%. Oral THC formulations have shown variable absorption and undergo extensive hepatic first-pass metabolism resulting in lower peak plasma THC concentration relative to inhalation and a longer delay (~ 120 minutes) to reach peak concentration. While the metabolic pathways differ slightly, CBD exhibits similar pharmacokinetics: Following oral administration of CBD, a similar plasma concentration-time profile to oral THC has been observed and documented (Lucas, 2017; Huestis, 2007). THC levels from smoking are much higher. Human clinical studies have shown bioavailability levels as high as 30% with peak plasma concentrations occurring 10 minutes after inhalation (McGilveray, 2005).
PHARMACOKINETICS
Pharmacokinetics refers to what happens to a substance from entering into the body until the exit of all traces. Drugs and supplements are introduced into the body primarily through the mouth (orally) but also through the skin (topically), rectum (anal), and intravenously (IV). Inhalation through smoke, vape or inhalers or nebulizers is also popular with cannabis oil. The focus of this article is the oral routes of administration. Pharmacokinetics, then, is the overall
study of the bodily absorption and distribution into the body of drugs. We will not be discussing Pharmacodynamics, which is the metabolism, utilization, and excretion of the cannabinoids in the body.
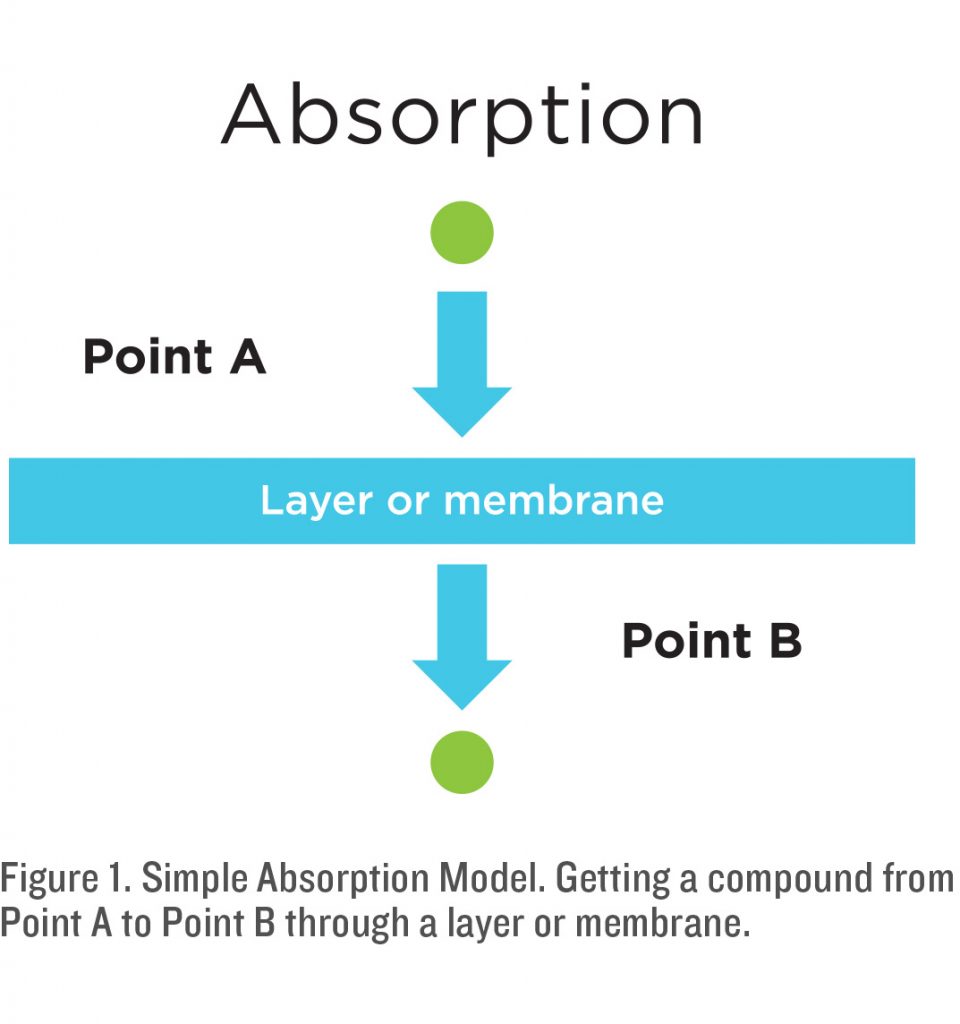
ABSORPTION
Absorption is defined here as the measurement of a compound through a cell wall layer inside the body or topically through the skin. Stated simply, it is the measurement of a compound to pass through a bio-layer.
BIOAVAILABILITY
Bioavailability is defined as the proportion of a drug or other substance which enters the circulation when introduced into the body and so it is able to have an active effect. In other words, bioavailability is the absorption of a drug or a supplement into the blood stream and utilization by body systems.
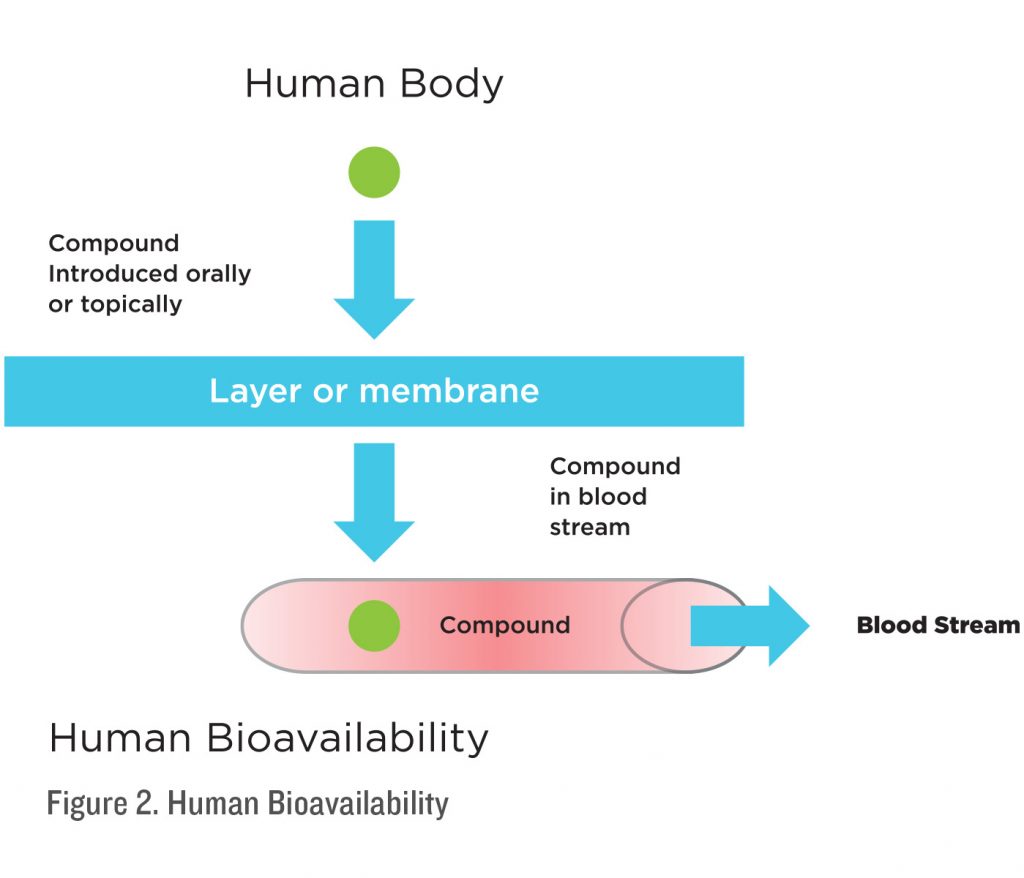
Absorption models will provide a good indication of how well compounds will pass through cell layers in ideal conditions. However, these models do not take into account the stability of a compound outside the body, in the mouth, stomach acid, or how it is broken down in the digestive system. It only tells us that in ideal conditions, a compound can pass through from point A to point B. Absorption also does not tell us how much of a compound is getting into the blood stream or being utilized by the body; it is however the first indicator that a compound is getting past the mucosal layer of the body. A compound that has poor absorption will not have good bioavailability. Conversely, a compound with good absorption
will generally have better bioavailability.
PURZORB® ABSORPTION STUDY
The purpose of the absorption study below is to identify and define full spectrum hemp oil and specifically indications of cannabidiol (CBD) bioavailability when a special absorption product is added. Understanding the pharmacokinetics of a drug is essential to understanding the onset, magnitude, and duration of its pharmacodynamic effects, maximizing therapeutic and minimizing negative side effects.
Increasing bioavailability of poorly absorbed compounds is an ongoing challenge in both pharmaceutical and nutritional science. Purzorb® is a proprietary micellization process which micellizes a decarboxylated full spectrum hemp oil mixture suitable for oral ingestion. According to the manufacturer each particle size is approximately 22 nm making it highly permeable in water. Animal models with Purzorb® have demonstrated a rapid and almost complete absorption (85%) in the intestinal lining using Franz diffusion apparatus (Davis, 2016).
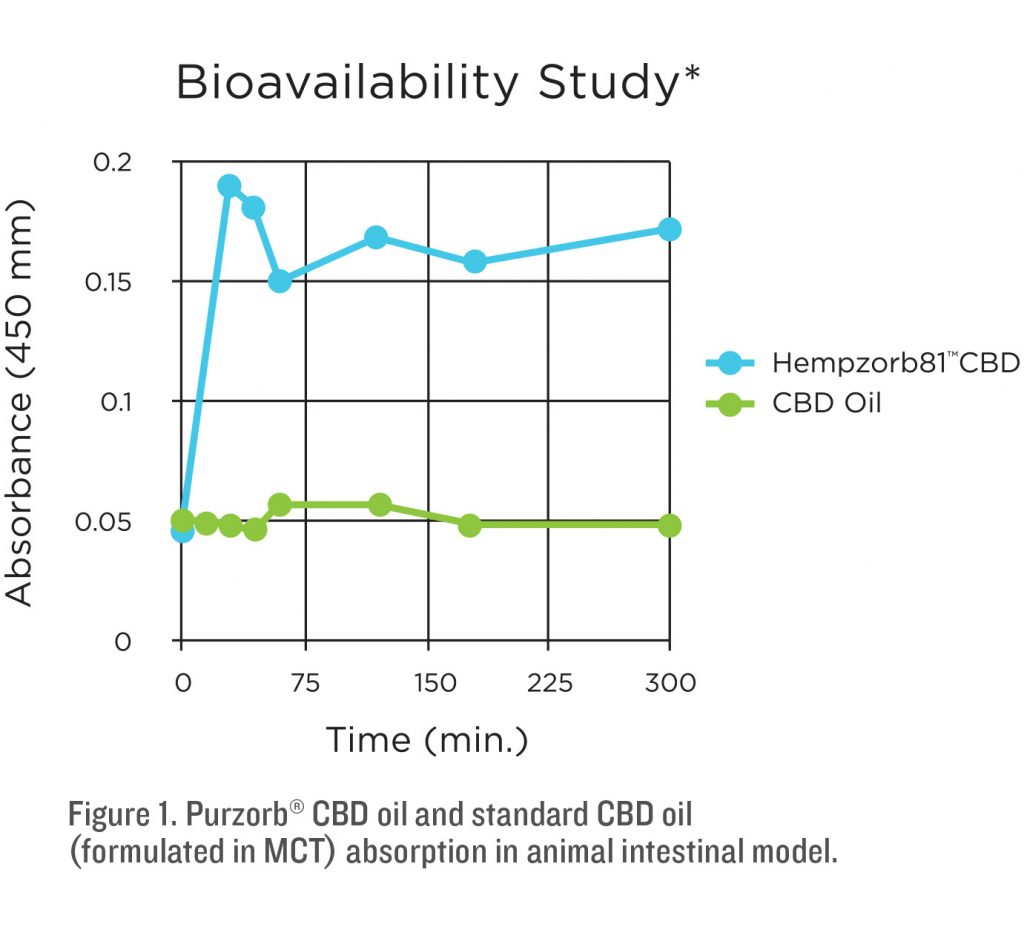
PURZORB® BIOAVAILABILITY STUDIES
Bioavailability is measured in the blood through high-performance liquid chromatography (HPLC; formerly referred to as high-pressure liquid chromatography). HPLC is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. Each component in the sample interacts slightly differently with the adsorbent material, causing different flow rates for the different components and leading to the separation of the components.
HUMAN STUDIES
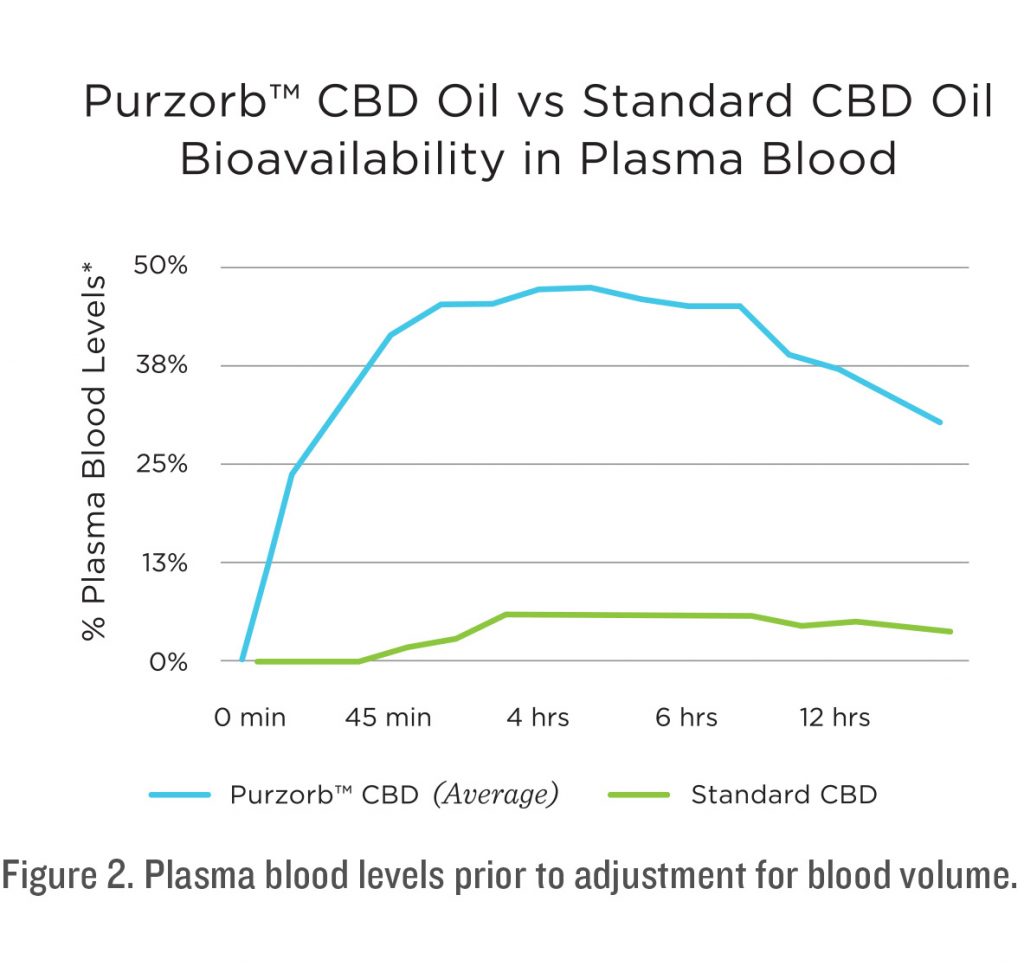
Two bioavailability studies evaluated the blood levels of Purzorb® orally administered to human subjects over a 12-hour period. Each time set had 3 vials of arterial blood drawn. Each sample was tested using HPLC. The report indicates the average of the three blood vials drawn and tested from each subject at each time point. Samples were drawn at Baseline and Post ingestion of product: 15 minutes; 30 minutes; 45 minutes; 1 hour, 45 minutes; 2 hours, 45 minutes; 3 hours, 45 minutes; 4 hours, 45 minutes; 5 hours, 45 minutes; 6 hours, 45 minutes; 7 hours, 45 minutes; 8 hours, 45 minutes; 9 hours, 45 minutes; 10 hours, 45 minutes; and 11 hours, 45 minutes.
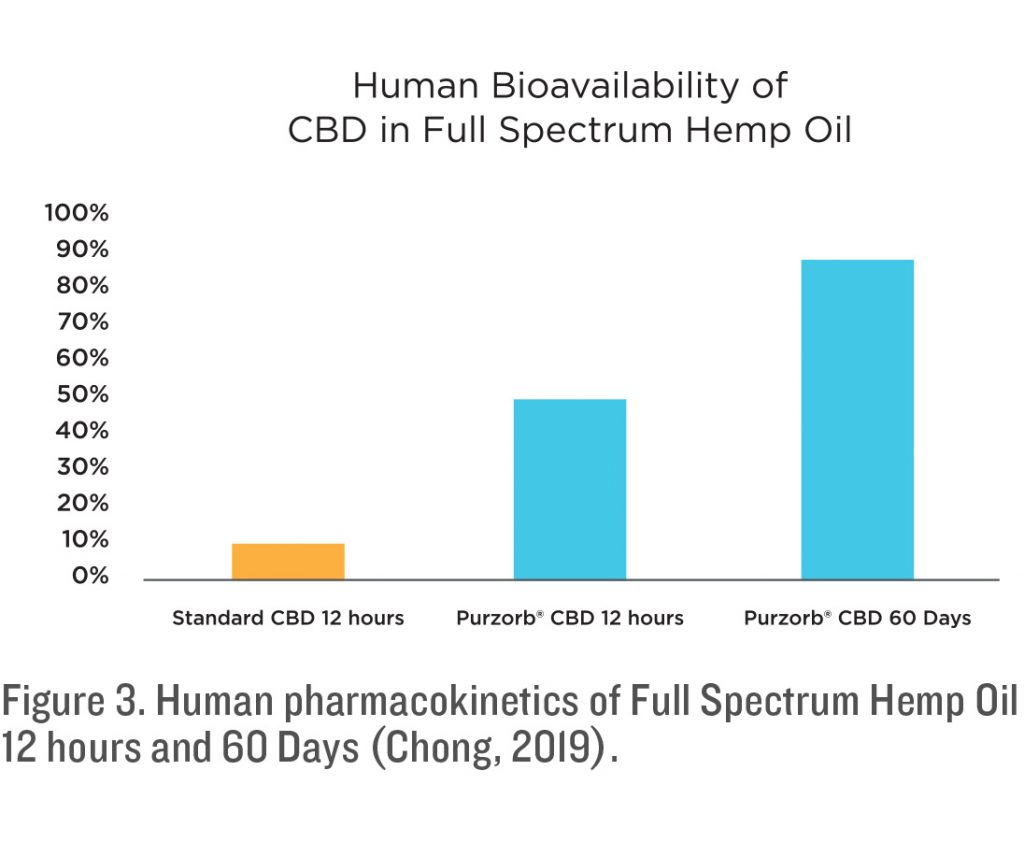
The two consecutive studies were carried out with fourteen subjects participating for a period of twelve hours. At the onset of these studies each subject had arterial blood drawn to set the Baseline of CBD in their bodies, each had a Baseline of 0.0 mg of CBD at Baseline. Each subject was given an oral dose of one vial with 8.95 mg of CDB. Vials were prepared by Med 7 and delivered to the researcher for use in this study. Each subject was given a vial and instructed to spray the liquid under their tongue and hold it there for 30 seconds before swallowing.
The nurse in attendance let each subject know when their 30 seconds was up so they could swallow the remaining liquid.
In a separate study of over 150 patients, Purzorb® was evaluated for safety and efficacy on several inflammatory, metabolic and cardiovascular markers. Patients were dosed orally with 3 mls a day of a Purzorb® finished product (Med 7®) in a tincture form. Each ml contained approximately 3.33 mg of CBD for a total of 10 mg a day. As part of this study, arterial blood was drawn at the end of the 60-day study and evaluated for serum concentration of CBD. The average bioavailability of Purzorb® patients was 86% (Chong, 2019). No other cannabinoids were measured, although it can be estimated that other cannabinoids such as THCV, CBG and CBN had similar pharmacokinetics (Grotenhermen, 2003).
CONCLUSION
The onset of Purzorb® is rapid and it has a lasting duration of CBD availability in the blood stream. All the patients were measured to have over 50% of the available CBD in their blood stream by the first measurement of 15 minutes. This exceeds what has been shown with CBD or THC that has been inhaled or vaped. The blood levels then measured significantly higher than what has been seen with standard CBD oil and other solubilizing methods. In the long-term study, CBD levels measure at over 85% of the oral dose. From these studies it is concluded that the uptake of Purzorb® CBD far exceeds the average uptake of standard CBD products available on the market today.
References
Gallily, R; et al. Overcoming the Bell-Shaped Dose-Response of Cannabidiol by Using Cannabis Extract Enriched in Cannabidiol. Pharmacology & Pharmacy, 2015, 6, 75-85
McGilveray, IA. Pharmacokinetics of cannabinoids. Pain Res Management (2005) 10 Suppl A: 15A-22A
Pertwee, R G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: ▲ 9-tetrahydrocannabinol, cannabidiol and ▲ 9-tetrahydrocannabivarin. Br J Pharmacology (2008) 153 (2): 199-215.
Grotenhermen F1. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. (2003) 42(4):327-60
Lucas, CJ; et al. The Pharmacokinetics and the Pharmacodynamics of Cannabinoids, Br J Clin Pharmacol (2018) 84 2477–2482.
Huestis, MA. Human Cannabinoid Pharmacokinetics, National Institute of Health Manuscript (2007) 4(8); 1770-1804.
Davis, P. The Bioavailability of Purzorb® CBD in Animal Intestinal Model. (2016) Unpublished abstract.
Chong, C; et al. Human Clinical Trial Evaluating the Safety and Efficacy of Purzorb® Full Spectrum Hemp Oil (Med 7). (2019) Unpublished manuscript.
©Med 7, LLC
All information is the property of Med 7, LLC. Any unauthorized reprint or use of this material without the express written permission from Med 7, LLC is strictly prohibited. www.med7cbd.com