Anti-Inflammatory Activity of a Full Spectrum CBD-Rich Hemp Formulation in Patients with COVID-19 Infection
Click here for study publication >
Growing evidence from multiple investigators, utilizing a broad array of hemp-based compounds spanning individual isolates to full-spectrum formulations, is developing an ongoing dictum of a role to be played by CBD and/or other cannabinoids or cannabinoid metabolites as a potential source of new treatments to control the COVID-19 pandemic spread. In our study, an early and one of the first human subject studies, substantial evidence has been developed to consider highly bioavailable CBD formulations, i.e. Hempzorb81™ as a potential adjuvant to agents available as preventative and therapeutic options for COVID-19 infections. We report analysis on a number of inflammatory molecules or markers associated with inflammatory pathways, including TNFα, CRP, IL-1, IL-6 and WBCs, whose expression are characteristic of the Cytokine Storm Syndrome, are significantly reduced in COVID-19 positive patients following institution of a Hempzorb81™ regimen.
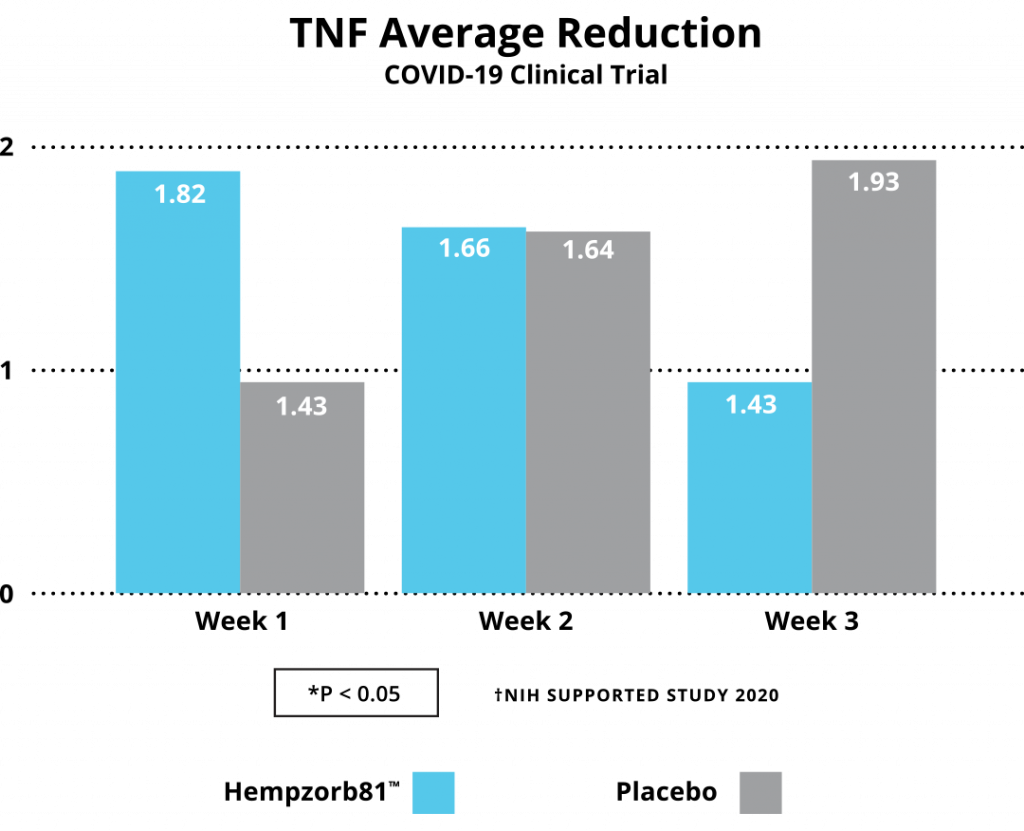
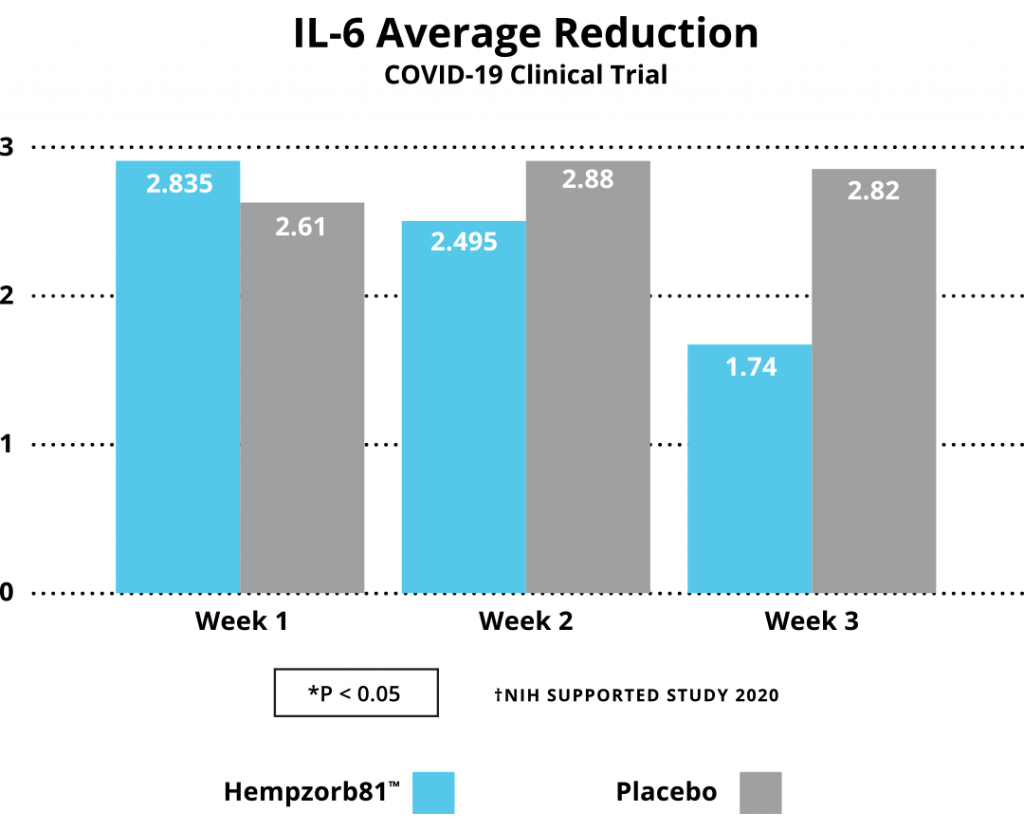
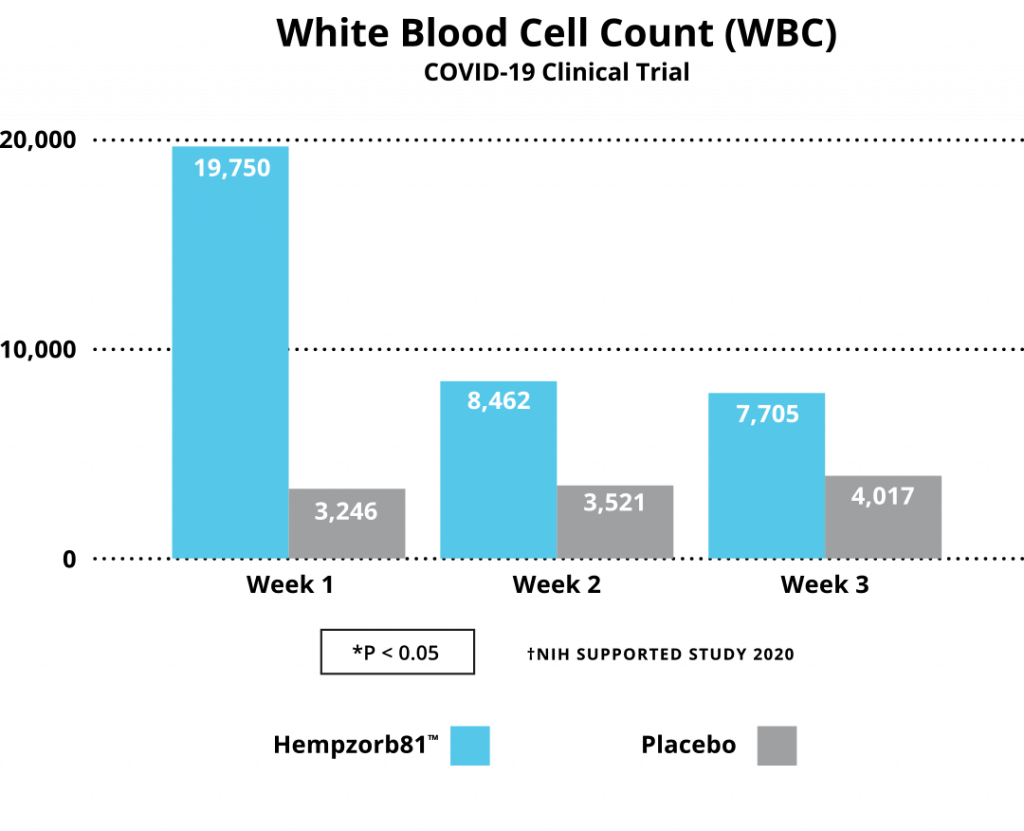

Together with the reduction in these anti-inflammatory markers, additional strong evidence for such a consideration include: (i) there were no reported side effects over the course of the studies; (ii) there were no drug-drug interactions identified; and (iii) the important fact that no patients withdrew from the study or were hospitalized as a result of the infection acquired during the study strongly indicates utilization of highly bioavailable CBD formulations and are gaining consideration as potential natural adjuvants in SARS-CoV2 treatment and prevention strategies. Finally, until widespread vaccination and therapeutics are available worldwide, a careful institution of hemp-based formulations are a consideration to potentially limit the spread and clinical course of the SARS-Co-V2COVID-19 pandemic in areas lacking access to such resources.